
The approval of the controversial Alzheimer’s drug of Biogen Inc by the FDA is an unprecedented decision that will improve treatment for the brain deteriorating condition, according to a report by Bloomberg.
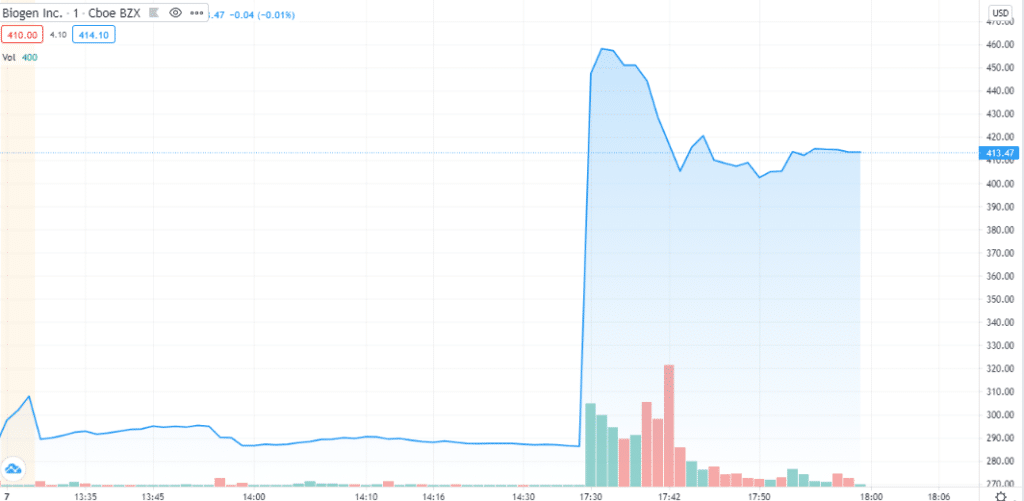
Biogen received an accelerated approval from the FDA, which demands for additional research to ascertain the benefits of the drug therapy to sustain it in the market.Alzheimer’s drug will be sold under brand, Aduhelm and an estimated price of $56,000 per year.
The trading of Biogen shares was stopped ahead of the landmark announcement. The share price increased by 55%.
The approval is one of the most influential FDA decisions in the efforts to provide medical help to millions of people diagnosed with Alzheimer condition.
Patient advocacy groups have voiced their support for the therapy despite concerns from some scientists.Physicians are concerned the private insurers and government Medicare program will not cover the cost for the Alzheimer’s drug.




